

http://pubs.acs.org/doi/abs/10.1021/ja105649g
Cloning and Elucidation of the FR901464 Gene Cluster Revealing a Complex Acyltransferase-less Polyketide Synthase Using Glycerate as Starter Units
Feng Zhang, Hai-Yan He, Man-Cheng Tang, Yu-Min Tang, Qiang Zhou, and Gong-Li Tang*
State Key Laboratory of Bio-organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
J. Am. Chem. Soc., 2011, 133 (8), pp 2452–2462
DOI: 10.1021/ja105649g
Publication Date (Web): February 3, 2011
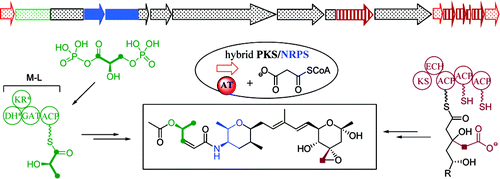
FR901464, an antitumor natural product, represents a new class of potent anticancer small molecules targeting spliceosome and inhibiting both splicing and nuclear retention of pre-mRNA. Herein we describe the biosynthetic gene cluster of FR901464, identified by degenerate primer PCR amplification of a gene encoding the 3-hydroxy-3-methylglutaryl-CoA synthase (HCS) postulated to be involved in the biosynthesis of a β-branched polyketide from Pseudomonas sp. No. 2663. This cluster consists of twenty open reading frames (ORFs) and was localized to 93-kb DNA segment, and its involvement in FR901464 biosynthesis was confirmed by gene inactivation and complementation. FR901464 is biosynthesized by a hybrid polyketide synthase (PKS)/nonribosomal peptide synthetase (NRPS), HCS, and acyltransferases (AT)-less system. The PKS/NRPS modules feature unusual domain organization including multiple domain redundancy, inactivation, and tandem. Biochemical characterization of a glyceryl transferase and an acyl carrier protein (ACP) in the start module revealed that it incorporates D-1,3-bisphosphoglycerate, which is dephosphorylated and transferred to ACP as the starter unit. Furthermore, an oxidative Baeyer−Villiger reaction followed by chain release was postulated to form a pyran moiety. On the basis of in silico analysis and genetic and biochemical evidances, a biosynthetic pathway for FR901464 was proposed, which sets the stage to further investigate the complex PKS biochemically and engineer the biosynthetic machinery for the production of novel analogues.
附件下载: