Total synthesis and synthetic organic chemistry as a whole provide society a fantastic return on investment with innumerable fundamental and applied tangible advancements. From a fundamental perspective, total synthesis is a barometer and proving ground for new methodologies and new strategies or ways of thinking. From an applied standpoint, having sustainable and reliable access to biologically active natural isolates can demystify new areas of biology or provide promising candidates for drug discovery.”
——Phil S. Baran
Despite the incredible methodological and strategic advances in synthetic organic chemistry, the efficient and scalable preparation of complex molecules (e.g., natural products) still presents a formidable challenge to the scientific community. Our research programs aim to provide innovative solutions through the development of simplifying methods and strategies. Our main concern has been to increase synthetic efficiency, typically by shortening synthetic routes, increasing overall yields, and improving scalability.
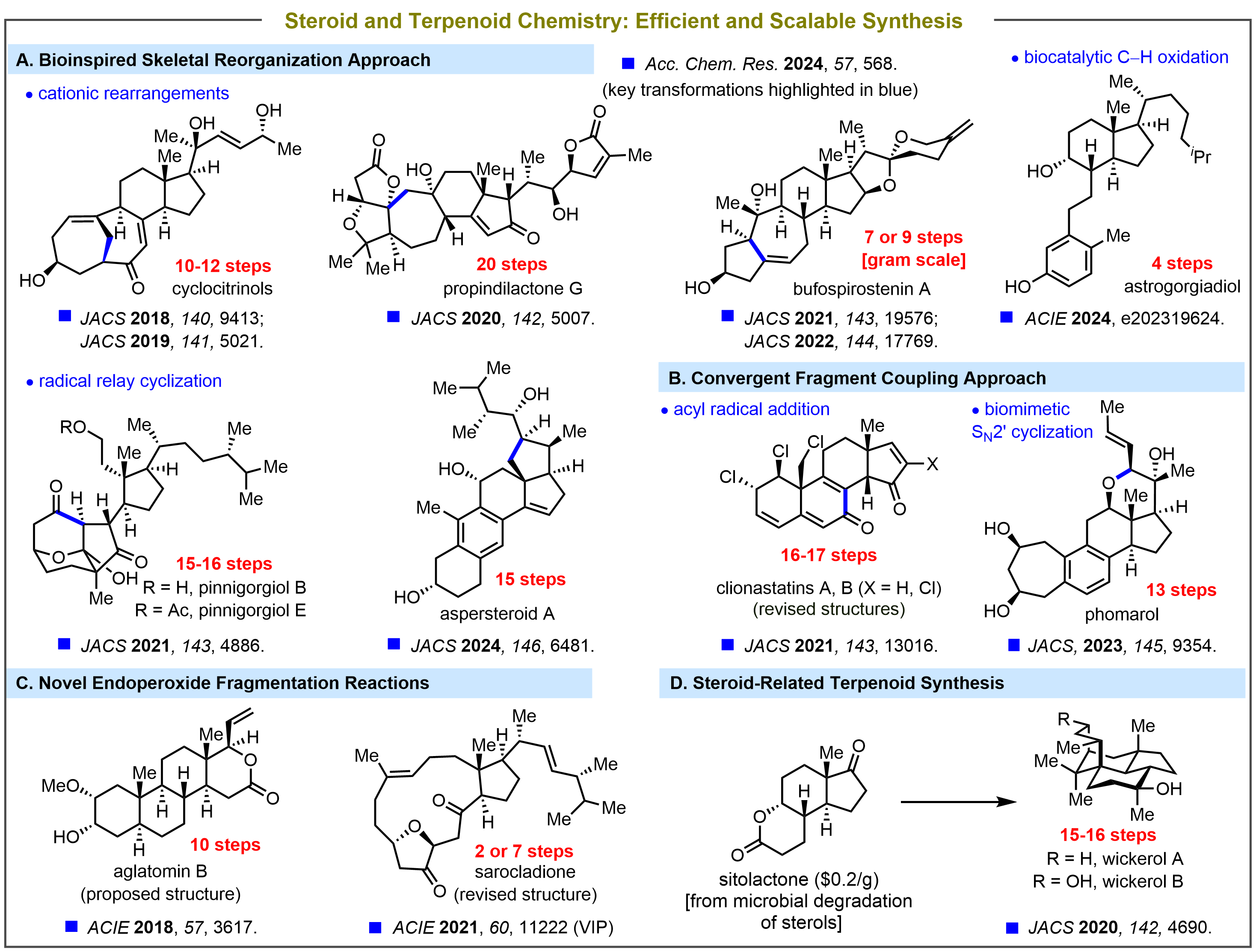
Inspired by Nature’s sophisticated synthetic machinery, we seek to identify and explore “bioinspired” strategic transformations that would lead to the rapid construction of the core framework of the target molecules from inexpensive, readily available starting materials. In addition to this bioinspired skeletal reorganization approach, we also develop several strategic C–C/C–O bond-forming reactions to quickly build the polycyclic ring systems from easily accessible building blocks via convergent fragment coupling. Another focus of our research is the development of novel synthetic methods to make or break chemical bonds which have practical and broad applications in organic synthesis.
Currently we are interested in the efficient synthesis of steroid and terpenoid natural products. The ultimate goal of this endeavor is to synthesize natural products and their synthetic analogues in useful quantities (scalable synthesis), thereby enabling full evaluations of their potential bioactivities.